
This blog post was created in collaboration with our partner Linxens Healthcare, a leading provider of electronic skin patches, biosensors, and connected solutions.
In the rapidly advancing world of healthcare technology, the transformation of biosignals into actionable clinical data is key to enhancing patient care and improving outcomes. With a growing demand for real-time monitoring, diagnostics, and data-driven therapy delivery, the ability to seamlessly capture, interpret, and transmit these signals is more critical than ever.
Linxens and Nocturnal have come together to offer integrated services that facilitate this process. This partnership leverages their combined expertise to simplify the path from biosignal acquisition to clinical data analysis, while accelerating device development timelines and minimizing the risks of delays and setbacks in the development process.
It All Starts with Quality Signal Acquisition
Biosignals, the measurable physiological signals produced by the body, offer a wealth of information about our health and well-being. Advances in sensor technology have enabled the capture of a diverse range of biosignals, opening new avenues for continuous health monitoring and personalized medicine.
Electrical signals, such as those generated by the heart, brain, and muscles, are among the most commonly captured biosignals. Electrocardiograms (ECGs) measure heart activity, providing insights into cardiac health and rhythm. Electroencephalograms (EEGs) record brainwave patterns, helping to diagnose neurological disorders and monitor brain function. Electromyograms (EMGs) assess muscle activity, aiding in the diagnosis of muscle diseases and the development of rehabilitation therapies.
Chemical signals, often measured through biosensors, offer valuable information about metabolic processes and overall health. Continuous glucose monitoring (CGM) systems, for example, track glucose levels in real-time, empowering individuals with diabetes to manage their condition effectively. Lactate monitoring can provide insights into muscle fatigue and exercise performance, while cortisol monitoring can help assess stress levels and overall well-being.
In addition to electrical and chemical signals, physical signals, such as body temperature and movement data, can also be captured by integrated sensors. Body temperature monitoring is essential for detecting fever and other health issues, while acceleration and posture data can be used for activity tracking, fall detection, and sleep monitoring. By combining these various biosignals, it is possible to gain a comprehensive understanding of an individual's health status and lifestyle patterns.
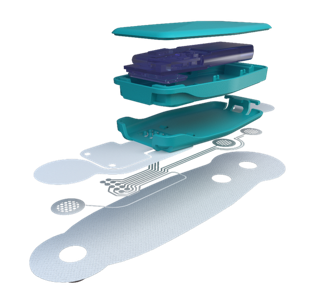
Image courtesy of Linxens
High Resolution, Low Noise, and Better AI Data
Once the raw data has been collected, the transformation from raw biosignals to clinical data relies on both hardware and software capabilities to ensure the delivery of accurate, meaningful, and actionable insights.
A major challenge in biosignal processing is noise—unwanted interference that can distort the signals being collected. Sources of noise may include electromagnetic interference, motion artifacts, or physiological processes that obscure the targeted signals. To ensure data accuracy, noise reduction techniques are applied during signal acquisition and processing. Sophisticated algorithms, filtering methods, and active noise-canceling technologies help eliminate these interferences while preserving the integrity of the core data.
By minimizing noise, medical devices can deliver cleaner, higher-resolution signals, making the analysis more reliable, particularly in devices where the distinction between a normal and abnormal signal is subtle, such as in cardiac monitoring or neurological assessments.
As AI becomes an integral part of nearly every medical device, the need for clean, high-quality data is more critical than ever. AI-driven diagnostic tools rely heavily on the accuracy of the input data to deliver reliable, actionable insights. Any noise or distortion in the biosignal data can lead to false interpretations or missed diagnoses. Therefore, ensuring low-noise, high-resolution data is essential for maximizing the potential of AI in medical diagnostics, enabling more precise decision-making and improving patient outcomes across a range of applications.
Power Efficiency in Signal Processing and Transmission
Power efficiency is a critical factor in the design of biosignal acquisition devices, especially wearable and implantable devices that rely on continuous, long-term monitoring. Not only must these devices acquire and process biosignals like ECG or chemical markers efficiently, but they must also transmit this data to external devices or healthcare systems without draining the battery quickly. The integration of ultra-low-power components and intelligent signal processing algorithms is key to ensuring these devices operate for extended periods without frequent recharging or battery replacements.
Hardware-based noise reduction, such as active noise-canceling circuits, plays a dual role in this process. It reduces signal distortion to enhance data quality while operating with minimal power consumption. In combination with optimized signal processing algorithms, these systems allow for the continuous monitoring of vital signs, such as heart rhythms or glucose levels, without compromising on battery life.
In addition to efficient signal acquisition, low-power data transmission is essential for preserving energy. Wireless communication protocols such as Bluetooth Low Energy (BLE) are commonly used in medical wearables to transmit data to external devices. These protocols are designed to use minimal power while maintaining reliable, real-time communication between the wearable device and the healthcare provider’s monitoring system.
For example, in ECG monitoring, once the data is acquired and filtered, the use of a low-power transmission method ensures that the patient’s vital signs are continuously sent to the healthcare provider without excessive battery drain.
Balancing power-efficient data transmission with the needs of real-time monitoring allows for longer operational cycles, which is essential for devices that are meant to be worn for days, weeks, or even months at a time. This balance between effective biosignal acquisition, noise reduction, and low-power data transmission ensures that the device remains functional and reliable over extended periods, enabling continuous, accurate data collection and improving overall patient outcomes.
End-to-End Safety and Compliance
Ensuring patient safety is a top priority in medical device design. Devices must not only be accurate but also comply with stringent regulatory standards. The end-to-end safety of a medical device involves robust testing, risk management, and quality assurance procedures.
In addition to safety features, regulatory documentation plays a crucial role in securing market approval for medical devices. Detailed records of the design process, testing protocols, and risk management strategies must be compiled and submitted to regulatory bodies to demonstrate that the device meets the required safety standards. Streamlining this documentation process helps accelerate time to market while ensuring compliance with all necessary regulations.
Documentation for Regulatory Approval
For any medical device, gaining regulatory approval is a pivotal milestone. Regulatory bodies like the FDA or EMA require detailed documentation that proves the safety, efficacy, and quality of the device before it can be brought to market. This process can be daunting, but thorough and accurate documentation can significantly streamline the approval timeline and reduce potential delays.
Documentation for regulatory approval includes extensive records of the device’s development lifecycle. This begins with the design and risk assessments, followed by testing data, quality assurance protocols, and clinical trial results. Each step must be clearly documented to demonstrate that the device and development process comply with medical industry standards, such as ISO 13485 for quality management systems and ISO 62304 for software development .
Additionally, the integration of biosensors, data processing algorithms, and communication protocols must be outlined, ensuring that the device not only meets functional requirements but also adheres to stringent safety regulations. For example, implantable devices must pass rigorous tests to confirm they do not cause adverse effects within the body, while wearables must prove that they can function accurately and safely in dynamic, real-world external environments.
Regulatory documentation also extends beyond the product launch. Post-market surveillance, ongoing risk management, and device updates must be documented to maintain compliance and ensure patient safety throughout the product’s lifecycle. By building a comprehensive documentation framework from the beginning, companies can navigate the regulatory landscape more efficiently, ensuring that their innovations reach patients faster.
Some Exciting Use Cases
Sweat Monitoring via Stick-to-Skin Patches
Linxens Healthcare possesses strong expertise in designing and manufacturing stick-to-skin patches and chemical biosensors. In combination with Nocturnal's advanced ultra-low-power hardware and software, Linxens’ technology enables innovative solutions for continuous health monitoring. By capturing and analyzing sweat biomarkers like sodium, potassium, lactate, and cortisol, patches can provide real-time insights into hydration status, electrolyte balance, muscle fatigue, and stress levels. This technology empowers individuals to optimize their performance, manage chronic conditions, and improve overall well-being through extended monitoring with minimal battery consumption.
Monitoring Cardiac Activity with ECG Stick-to-Skin Patches
Linxens’ industrial capabilities in design, functional printing, and assembly of stick-to-skin patches make it possible to reliably capture electrical signals from the heart in ambulatory applications. Combining these patches with ultra-low power hardware and software designed by Nocturnal to condition and analyze biosignals enables accurate detection of cardiac rhythms over extended monitoring periods using readily available batteries.
Temperature Monitoring in Clinical and Remote Settings
Linxens can incorporate vital sign sensors into their patches through the use of both functional printing and traditional circuit assembly. These capabilities allow small, inexpensive stick-to-skin patches to reliably detect changes in skin temperature, offering valuable information for health monitoring. By combining these features with Nocturnal-designed ultra-low-power hardware and software, continuous extended temperature monitoring is practical even in remote or clinical settings.
The Future of Biosignal Integration
The Linxens Healthcare - Nocturnal collaboration provides a comprehensive end-to-end design and manufacturing service for wearable health device development, streamlining the process from sensor integration to data analytics and regulatory compliance.
The combination of these innovative technologies enable the capture of low-noise, high-resolution biosignals using minimal power, allowing for better signal quality over longer periods. By ensuring the accuracy of data, extending the operational life of devices, and streamlining regulatory approval, these key elements form the foundation for effective biosignal monitoring and analysis.
To learn more about how this collaboration is driving innovation in healthcare, visit Linxens and Nocturnal.
Additional Resources
MOST READ POSTS
Insights from a decade of milestone-based projects
Transforming biosignals into clinical insights
Why investors say No to medical device startups



