Embedded software:
The brain behind your device
Embedded software is the key to making your medical device smarter, more responsive, and more effective.
Our software engineers are always two steps ahead of the latest advances in software development for Class II/Class III devices, including cardiac rhythm management, neuromodulation, and other implantable devices.
- Power management: Embedded software is the most critical component of a low-power device. Properly architected software can optimize the use of battery power, extending the life of implantable devices and reducing the need for frequent charging or replacements.
- Real-time monitoring: Advanced sensing and data collection enable continuous monitoring of patient biosignals and other clinical indicators, allowing the device to respond in real-time and alert a medical provider to changes in the patient’s condition.
- Adaptive algorithms: Advanced algorithms can help the device learn from patient data and adapt to their needs over time.
- Security: As medical devices become more connected, advanced encryption, authentication, and other security safeguards are critical to the protection of patient data and privacy.
Code that will scale with your product roadmap
Good code will make your device work as expected today. Great code will be designed to support the functionality you may want to add in the future — whether it’s a new hardware component, additional sensors, or new analytic capabilities.
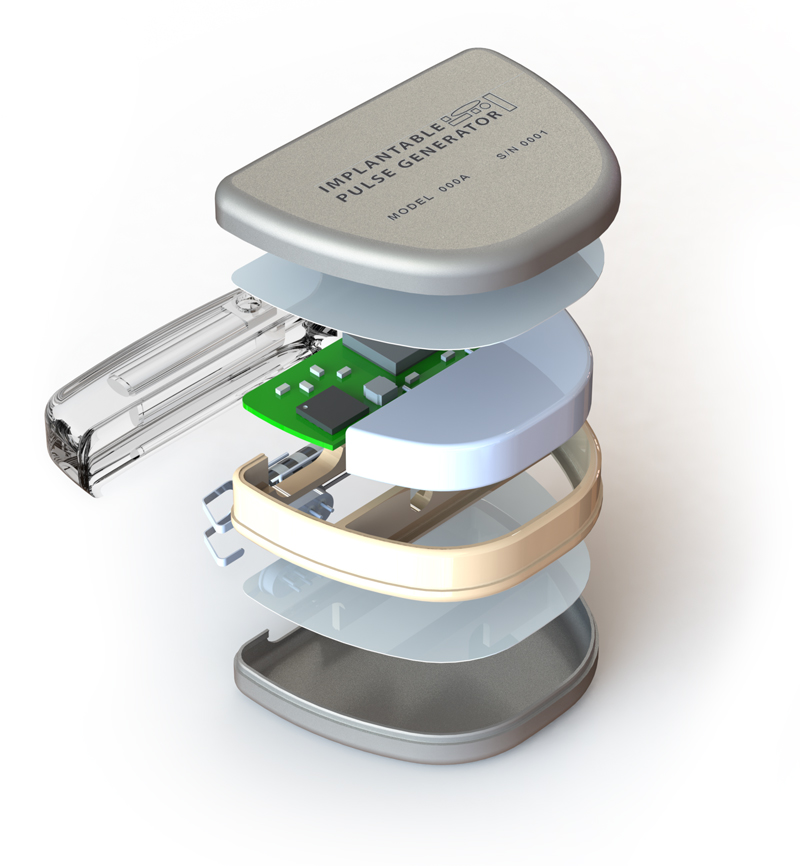
There is more to it than just code: Software Quality Management Systems
When it comes to medical device software, getting the code to make the device function is just part of the picture. To support regulatory submission, our team will check all the boxes ensuring the design and development process is documented in compliance with ISO 13485:2016, IEC 62304, ISO 14971, and any applicable regulatory standards.
Result-based business model
Cash efficiency is a “do or die” proposition for any early stage company, and hourly-priced projects can quickly lead to cost and time overruns.
Our pricing is based on results, giving you the predictability you need to plan your budget and meet your funding milestones with confidence.
And with direct access to our project management system, you have real-time visibility into our progress so you always know where you are.
design for success
From napkin sketch to commercially-viable product, we take a holistic approach to product development, addressing all the required aspects for your device to be successful:
Technical
Ensuring the device can perform as required to deliver the intended clinical results
Usability
Ensuring the device can be operated in the intended clinical setting
Standards
Ensuring the design and development process is documented in compliance with ISO 13485:2016 and that the design meets the requirements imposed by relevant regulatory standards
Manufacturability
Ensuring manufacturing is feasible within your business case assumptions and identifying qualified suppliers and manufacturing partners
Financial
Optimizing device cost of goods sold (COGS)
“The re-architecture of the imaging processing and analysis algorithms produced real-time analytics at 15 fps (down from 15 sec), integrated within a scalable software platform developed by Nocturnal that demonstrates supports our expanded product roadmap.”
Bruce Ferguson, MD, Chief Medical Officer & Co-Founder, Perfusio


