Design Your Device
to Deliver AI-Ready Data
The success of AI applications depends on the data feeding the algorithms. More data isn’t always better—what matters is high-quality, clinically relevant signals collected efficiently and securely.
To accelerate time to market and reduce risk, tap into proven expertise for your AI data pipeline—so your team can focus on your algorithms and your competitive edge.
With our pre-certified biosensors, proven hardware and software building blocks, and milestone-based pricing, you can get to market faster, at lower risk, and with capital preserved for growth.
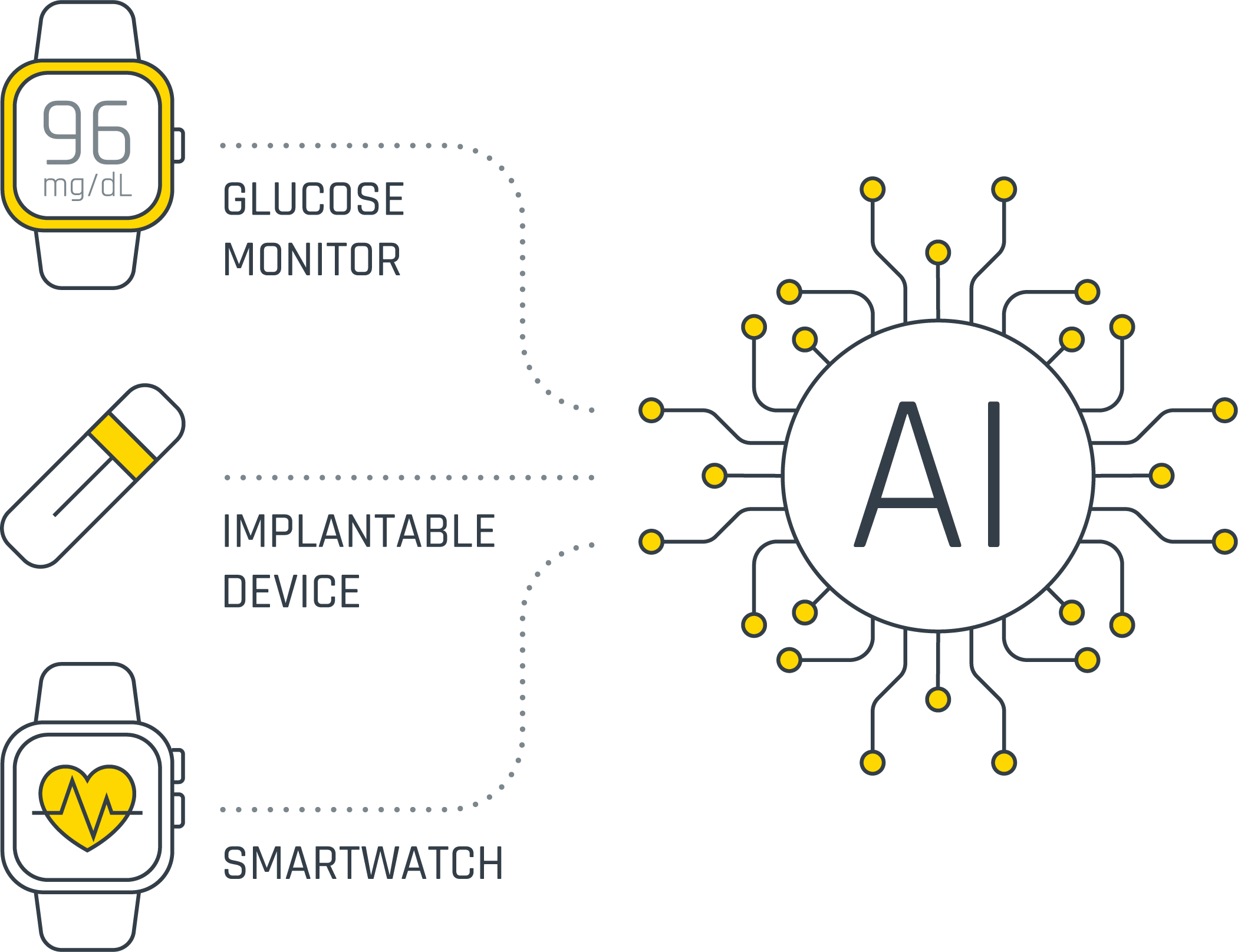
TAP INTO PROVEN EXPERTISE IN AI-READY DATA
Building the data pipeline that feeds your algorithms is a massive undertaking. From signal acquisition and power management to secure data transmission and compliant cloud storage, every piece of the pipeline demands specialized expertise, acquired over years of continuous iterations and optimization.
FAQ
Result-based business model
Cash efficiency is a “do or die” proposition for any early stage company, and hourly-priced projects can quickly lead to cost and time overruns.
Our pricing is based on results, giving you the predictability you need to plan your budget and meet your funding milestones with confidence.
And with direct access to our project management system, you have real-time visibility into our progress so you always know where you are.
design for success
From napkin sketch to commercially-viable product, we take a holistic approach to product development, addressing all the required aspects for your device to be successful:
Technical
Ensuring the device can perform as required to deliver the intended clinical results
Usability
Ensuring the device can be operated in the intended clinical setting
Standards
Ensuring the design and development process is documented in compliance with ISO 13485:2016 and that the design meets the requirements imposed by relevant regulatory standards
Manufacturability
Ensuring manufacturing is feasible within your business case assumptions and identifying qualified suppliers and manufacturing partners
Financial
Optimizing device cost of goods sold (COGS)
“In just one year, Nocturnal took our implantable cardiac monitor from a concept to a fully functional device.”
Jaeson Bang, Founder & CEO, Future Cardia


